
|
|
The Galvanizing Process Surface Preparation Surface Preparation is the most important step in the application of any coating. In most instances where a coating fails before the end of its expected service life, it is due to incorrect or inadequate surface preparation. The surface preparation step in the galvanizing process has its own built-in means of quality control, in that zinc simply will not react with an improperly cleaned steel surface. Any inadequacies in surface preparation are immediately apparent when the steel is withdrawn from the molten zinc, allowing for immediate corrective action. Surface preparation for galvanizing typically consists of the following three steps: Caustic Cleaning – A hot alkali solution is often used to remove organic contaminants such as dirt, paint markings, grease and oil from the metal surface. Epoxies, vinyls, asphalt or welding slag are removed via grit-blasting, sandblasting or other mechanical means. Pickling – Scale and rust normally are removed from the steel surface by pickling in a dilute solution of hot sulfuric acid or ambient temperature hydrochloric acid. Fluxing – Fluxing removes oxides and prevents further oxides from forming on the surface of the metal prior to galvanizing. It also promotes bonding of the zinc to the steel or iron surface. In the dry galvanizing process, the steel or iron materials are dipped or pre-fluxed in an aqueous solution of zinc ammonium chloride. The material is then thoroughly dried prior to immersion in molten zinc. 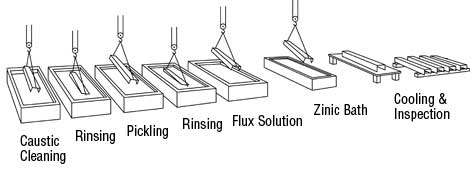 |